[nomedia="http://www.youtube.com/watch?v=OgWAghSdnZo"]YouTube - from boiling water to snow in less than a second in winter in New York[/nomedia]
why does this work?
I just tried it with boiling water (works) then with room temp water (doesn't work)
Results 1 to 23 of 23
Thread: boiling water to snow.... why???
-
01-31-2011, 08:32 PM #1
boiling water to snow.... why???

-
01-31-2011, 08:39 PM #2
Not a thermal engineer but would venture to say it's since boiling water is close to changing stage to gas. Three stages of matter solid, liquid and gas. As a material approaches change state it isin flux.
Just a guess, but it's pretty cool and I will try it next time I'm in snow. Curious is it below freezing when you are experimenting?I need to go to Utah.
Utah?
Yeah, Utah. It's wedged in between Wyoming and Nevada. You've seen pictures of it, right?
So after 15 years we finally made it to Utah.....
Thanks BCSAR and POWMOW Ski Patrol for rescues
8, 17, 13, 18, 16, 18, 20, 19, 16, 24, 32, 35
2021/2022 (13/15)
-
01-31-2011, 08:41 PM #3
-20'C out my back door right now....

-
01-31-2011, 08:42 PM #4
google "Mpemba effect"
-
01-31-2011, 08:44 PM #5
-
01-31-2011, 08:47 PM #6
Water is much less dense in vapor form than in liquid form. My best guess is that in this less dense form its much more able to loose all of it's heat than it would in the normal liquid form. IE: It's easier to cool down 155 12oz bottles of beer than a single keg of beer, if you were to just put them outside in the cold.
<p>
go Go GO! 24-25: 104! [SIZE="1"]23-24: 75. 22-23: 56. 21-22: ?. 20-21: 10+?. 19-20: 79. 18-19: 86. 17-18: 80. 16-17: 56. 15-16: 40. 14-15: 33. 13-14: 56ish. 12-13: 51. 11-12: 65. 10-11: 69. 09-10: 65.[/SIZE]</p>
-
01-31-2011, 08:47 PM #7
[ame]http://en.wikipedia.org/wiki/Mpemba_effect[/ame]

-
01-31-2011, 08:48 PM #8
Near boiling water freezes quicker than room temperature water. I remember that from a argument with a physics geek. Can't remember why, but it'd related. ;-) Probably due to the extra movement of molecules in boiling water which exposes more molecules to the surface (and thus cold)...
-
01-31-2011, 10:53 PM #9
This is not the mpemba effect.
First, realize that you are viewing low rez. When you view high contrast or high rez of such experiments, there is generally a large amount of droplets hitting the ground unless the temp is VERY low like -50C.
Most of the videos are -30 or -40.
Why does it work well with boiling water? Because the boiling water adds water vapor to the surrounding air (heated at the interfaces) and thus that interface air has a high relative humidity and high moisture capacity. The air is then rapiidly cooled making the air supersaturated and so the now freezing water vapor precipitates out: "snow"
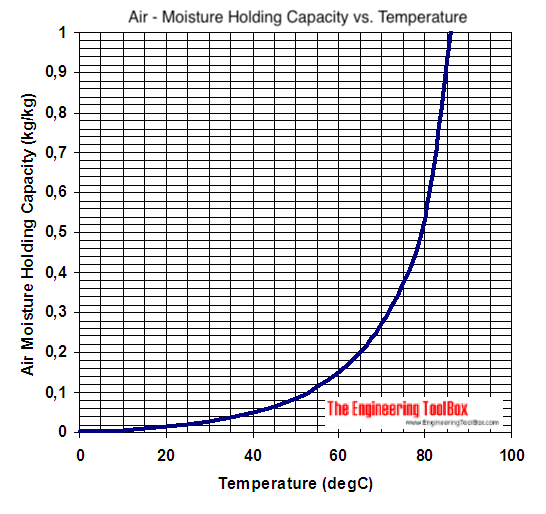
(warm air holds a SHIT TON more water vapor than cold air... which is why you lose water so fast in cold climates by breathing in the cold air even though the relative humidity outside might be 100%) Originally Posted by blurred
Originally Posted by blurred
-
02-01-2011, 11:07 AM #10
I tried this yesterday at -20'C, 250ml of boiling water (well just out of the kettle) and threw up the water over the back deck. No droplets of water hit the deck (snow, or vapor only, no ice stuck to the deck)
then used room temp, tap water and I can still see the ice on the deck, it all fell as water....
still not 100% sure why this works, but cool that it does....

-
02-01-2011, 11:42 AM #11
listen guys... a plane will totally be able to take off on a treadmill...
 www.dpsskis.com
www.dpsskis.com
www.point6.com
formerly an ambassador for a few others, but the ski industry is... interesting.
Fukt: a very small amount of snow.
-
02-01-2011, 01:43 PM #12
 Registered User
Registered User
- Join Date
- Oct 2003
- Location
- CO
- Posts
- 570
When water is boiling, all the water in the pot is at the boiling point, but needs a surface for phase change. Throwing it in the air increases surface area and causes the water to flash into gas phase, so you have smaller water droplets then in room temp water.
Small water droplets have a much greater surface area then large water droplets so freezing happens more readily, creating seed crystals.
Energy transfer occurs more rapidly over steeper gradients, so hot water cools more rapidly then cold water.
I think their may be pockets of varying pressure due to the flashing too, which aid in reaching energy of crystalization , but not sure about that.
-
02-01-2011, 02:03 PM #13
I'm calling bullshit on that. I'll bet that's baby powder. Taking a cup of boiling water and tossing it in the air does not produce snow.
-
02-01-2011, 02:32 PM #14
 Registered User
Registered User
- Join Date
- Mar 2010
- Posts
- 156
I just tried it at 19f/-7c and it works
-
02-01-2011, 03:34 PM #15
"Energy transfer occurs more rapidly over steeper gradients, so hot water cools more rapidly then cold water."
that doesn't add up as sure the bigger then gradients or difference the more heat is transferred. That is until the point when enough heat has been transferred so the gradient is lower and heat is tranferred at the same rate. Not that it would be able to catch up but if it did.
What I remember from school at the same time we were discussing why water is most dense at 4°C. There are ice crystals floating around in water before you get to 0°C. As the temperature decreases more ice crystals are formed and these crystals are less dense than liquid water. Ice floats. At the same time the liquid water is getting more dense as with almost all things when liquids get colder they get more dense. At 4°C the density increases is overcome by the density decreases.
So we were told the reason why warmer water will freeze faster is that warmer water doesn't have all these tiny crystals floating around so when subjected to cold the ice crystals can grow very quickly without running into any crystal fragments that would stop the growth.
Though I don't the above explains it all. The drops in the air would cool rapidly to do evaporative cooling plus they would shrink do to the mass lost to evaporation. So the resulting droplets would be smaller with more surface area and then again cool quicker than the larger droplets...
-
02-01-2011, 06:00 PM #16
^^^this. You are basically playing with RH and wet bulb over a very small period of time. Boiling water is thrown into cold air, vaporises, once vaporised the cold air cannot contain said water vapor, poof, precipitation. In the case of room temp water you just don't get the dispersal/vaporisation effect.
I do have a bit of trouble understanding how the nucleation process works through this though, usually you need specks of dust or other such materials to form the nucleus of a frozen droplet. Especially in your case, jaredstrauss, at -7C, as I believe water has trouble forming it's own standalone nucleus until -30 or so Celcius...
Anyone able to chime in on that side of this phenomenon?
I suspect the tiny water vapor molecules/droplets/whatever you want to call them become so small they can basically fuse with whatever tiny dust particles or other tiny debris might be in the air already but just speculation...
-
02-01-2011, 08:00 PM #17
what summit said... I'm a physics major, and the best explanation i can think of has to do with the hot water vaporizing faster than the cold water due to the extreme vapor pressure difference between the hot water and cold air. Not only is the air cold, it is extremely dry, meaning the hot water will vaporize much quicker when hot. Since the air is extremely cold, it can't hold as much water so it condenses, freezes, and falls as snow (think dew point, frost etc) Water cools much faster in its gas form and is able to reach a freezing temperature quicker than its liquid counterpart. The key here is that the hot water vaporizes faster due to the higher temp/saturation than the luke warm water.
-
02-02-2011, 07:24 AM #18
 Registered User
Registered User
- Join Date
- Oct 2003
- Location
- CO
- Posts
- 570
You're right in regard to temperature (ie the measure of the average kinetic energy), but molecules don't have temperature, they just have a KE. Temperature will decrease in a mostly continuous fashion, but energy transferred at collision is discrete so its not subject to this gradient decay. Although now I see my error using gradient to describe collisions, poor word choice. I was trying to simplify the concept.
The references to relative humidity are a good analogy, but it is actually the same phenomenon viewed from a different perspective. It isn't really why it works.
You need vapor or very small droplets to start crystal formation, the boiling water achieves this, and room temp water does not. The vaporization acts like the nozzle on a snow gun.BEWARE OF FEMALE SPIES
-
02-02-2011, 10:18 AM #19
 Registered User
Registered User
- Join Date
- Dec 2006
- Location
- Colorado
- Posts
- 195
Here's an explanation for the boiling water.
All things being equal, cold water freezes faster.
It takes time for the energy contained in a hot object to be transferred to a cold object. However, the rate of heat transfer is proportional to the temperature difference between the two objects, so hot water will lose heat faster than cold water. In other words, if you have water at 90 degrees C and water at 10 degrees C and the freezer is at -10 degrees C, the hot water will lose heat five times faster than the cold water; however, the cold water will still win the race. As the hot water cools itís rate of heat transfer will decrease, so it will never catch up to the cold water.
Some people claim that hot water freezes faster because a pot of boiling water can be thrown into the air on a cold winter day, and it freezes in mid air creating a shower of ice crystals. Whereas a pot of cold water thrown into the air comes down as large blobs of water. This happens because the hot water is so close to being steam, that the act of throwing it into the air causes it to break up into tiny droplets. (hot water is less viscous than cold water, listen to the sound it makes when you pour it in the sink) The small water droplets have a large surface area which allows for a great deal of evaporation, this removes heat quickly. And finally, the cooled droplets are so small, that they can be easily frozen by the winter air. All of this happens before the water hits the ground. Cold water is thicker and stickier, it doesnít break up into such small pieces when thrown into the air, so it comes down in large blobs.
-
02-02-2011, 03:52 PM #20
I can see a certain % evaporating very quickly , the water droplet heating up the air around it and alot of the water evaporating in the low RH air. But the remaining water would cool down as it provided the latent heat of evaporization. The evaporated water vapor would then cool and condense and form snow.
The remaining water droplets would be small enough , started off small and got smaller by losses to evaporization, that they would fall much more slowly , be mist, and with high surface are to mass ratio be able to form crystals.
-
02-02-2011, 10:00 PM #21
 Registered User
Registered User
- Join Date
- Mar 2009
- Location
- Aspen, Colorado
- Posts
- 2,644
I read about this last night and tried it this morning. It was -23F this morning, and very little of a quart of boiling water made it to the ground. There was a surprising amount of white vapor which drifted off. Very cool experiment. I dumped a pot of cold water out and it just splashed into the snow
-
02-04-2011, 03:21 PM #22
Discovery Channel weighs in:
http://news.discovery.com/videos/ear...explained.html
-
02-04-2011, 10:13 PM #23
This is sort of what I was driving at with nucleation in a roundabout sort of way. You need to break water particles into small enough pieces to create a frozen nucleus, around which other water particles can bind to it and freeze.
With a snow gun, it's compressed air being forced through a water-fed nozzle that does the breaking up and vaporising, alowing a nucleus to be created for snow crystals.
In a cloud, from my understanding, temps are often not low enough to crystalise a water droplet, so much of what forms can have dust particles as their nucleus.


 Reply With Quote
Reply With Quote








Bookmarks